Stpm Chemistry Experiment 2 Volumetric Analysis Acid Base And Redox Report
Volumetric analysis acid base and redox lab 1 this is a lab report for a physics experiment on simple harmonic motion.
Stpm chemistry experiment 2 volumetric analysis acid base and redox report. Workers essay by richard rodriguez. Volumetric analysis stoichiometry. V is the volume of the titrant which is used typically in liters.
Chemistry s title of experiments and project for stpm kerja kursus first term. Volumetric analysis acid base and redox. Volumetric analysis acid base and redox.
Here we are thinking about using a titration to determine the stoichiometry of a reaction. Stpm chemistry practical experiment 2 2012 free download as pdf file pdf text file txt or read online for free. Stpm chemistry coursework project pbs sample.
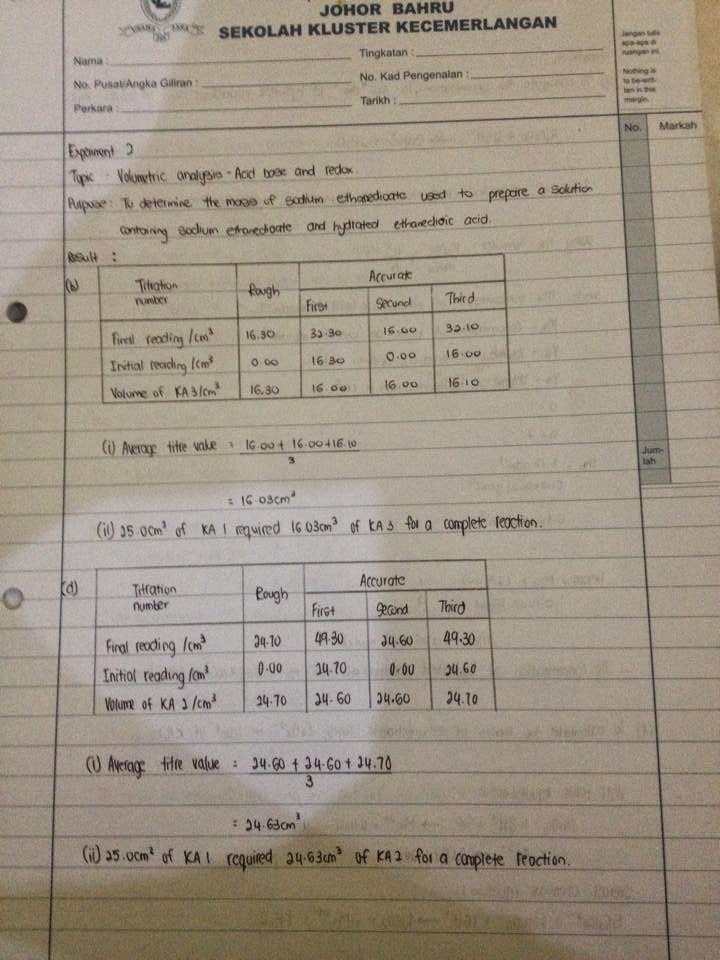
To determine the mass of sodium ethanedioate and hydrated ethanedioic acid. To determine the exact concentration of a monobasic acid hx. Similarly in redox system a titration method.
6 2 0 methodology 2 1 theory vitamin c can be determined by acid base reaction or oxidation reduction. Test for anions and cations no title. Chemistry s title of experiments and project for stpm.
In the acid base system a titration method helps in finding out the strength of one solution against another solution by the use of the ph sensitive indicator. M is the mole ratio of the analyte and reactant from the balanced equation. Volumetric analysis 3 redox titrations the point of this blog is to introduce you to redox titrations since the titration technique is not restricted to acid base reactions.
Stpm chemistry notes and questions please go to this site for more easy diagram for. To determine the mass of sodium ethanedioate and hydrated ethanedioic acid. Volume 74 issue 2 may 2006 p.
Many non acid base titrations are needed a constant ph throughout the reaction. To determine the exact concentration of a monobasic acid hx. Acid base titration and volumetric analysis the purpose of this experiment is to determine the naoh of a solution by titrating it with standard hcl solution to neutralize a known mass of an unknown acid using the naoh solution as a standard to determine the moles of naoh required to neutralize the unknown acid and to calculate the molecular mass of the unknown acid.
V is the volume of the analyte typically in liters. Physical quantities and units 2. Kedah trial 2009 chemistry p1 p2 p3 marking scheme sbp spm trial 2009 chemistry paper 1 sbp spm trial 2009 chemistry paper 2 sb.
Analysis of l and d ascorbic acid in fruits and fruit drinks by hplc.